
Our Research
Advancing infectious disease control through innovative solutions. Focus areas include Malaria Vaccine Discovery & Development, Improving Maternal and Neonatal Health, Development of Diagnostic and Surveillance Tools, and a Collaborative Approach to Science.
Research Overview
We are dedicated to advancing the field of infectious disease control through groundbreaking research and innovative solutions. Our primary focus areas include developing novel vaccine candidates for malaria, improving diagnostic and surveillance tools, and enhancing maternal and neonatal health in malaria-endemic regions. By leveraging cutting-edge technologies and a deep understanding of disease mechanisms, we aim to address some of the most pressing health challenges faced by populations in Africa, South America, Oceania, and Asia. Our work not only contributes to scientific knowledge but also aims to bring tangible benefits to global health through improved prevention, diagnosis, and treatment strategies.
1. Malaria Vaccine Discovery & Development
a. Identifying Novel Vaccine Antigens for Malaria
Our research focuses on the identification of novel vaccine antigens to develop more effective malaria vaccines. By leveraging wheat germ cell-free protein synthesis, we produce candidate recombinant malaria vaccine antigens from malaria parasite RNA. Our efforts have significantly advanced the discovery of new malaria blood-stage vaccine candidates and the secretome profiles of P. falciparum through immuno-screening approaches.
Key Contributions:
- Successfully expressed, validated, and assayed over 3,000 malaria parasite antigens.
- Evaluated the immunogenicity and vaccine potential of these antigens using serum samples from cohorts in malaria-endemic regions.
- Discovered that a high proportion of recombinant malaria proteins are recognized by antibodies in sera from malaria patients, with serum recognition correlating with protection against clinical malaria.
These findings are crucial because malaria remains a leading cause of death among children in Africa. With only two licensed malaria vaccines—one of which has limited efficacy—there is a pressing need to expand the pipeline of candidate malaria vaccines. Our research aims to develop second-generation vaccines that are more effective and have fewer side effects. Our work contributes to the global fight against malaria by paving the way for the development of more effective vaccine candidates, ultimately aiming to reduce the burden of this devastating disease.
b. Addressing the Global Challenge of Plasmodium vivax
Plasmodium vivax is the most widespread human malaria-causing pathogen, putting 2.5 billion people at risk across Africa, South America, Oceania, and Asia. Despite this, efforts to control P. vivax malaria lag significantly behind those targeting P. falciparum, particularly in vaccine development. Currently approvedP. falciparum vaccines do not protect against P. vivax. The World Health Organization’s Malaria Vaccine Technology Roadmap to 2030 aims for a P. vivax vaccine with 75% efficacy.
Kenya is a crucial location for studying malaria due to the increasing prevalence of P. vivax, traditionally rare in sub-Saharan Africa. Studies have shown P. vivax circulating in Turkana County, despite the belief that the Duffy blood group—which confers resistance—is absent in the local population. The presence of P. vivax poses a significant threat to malaria elimination due to its dormant liver stage (hypnozoites), which can relapse and sustain transmission. Moreover, the invasive mosquito vector Anopheles stephensi, recently identified in Kenya, thrives in urban areas and efficiently transmits both P. vivax and P. falciparum. This could drastically worsen malaria transmission.
Key Contributions:
- Participated in genome-wide research efforts to identify novel P. vivax malaria vaccine candidates.
- Employed immune-screening techniques to discover and evaluate potential vaccine candidates.
Our research addresses critical gaps in the knowledge and development of a P. vivax vaccine, contributing to global efforts to control and eventually eradicate P. vivax malaria.
c. Functional Characterization of Candidate Malaria Vaccines
Increasing the Portfolio of Functionally Characterized Candidate Plasmodium falciparum Malaria Vaccines
Our research aims to significantly expand the portfolio of functionally characterized candidate malaria vaccines, with a specific focus on Plasmodium falciparum. This endeavor involves identifying and evaluating new vaccine candidates, enhancing our understanding of their mechanisms of action. By targeting Plasmodium falciparum, the most deadly of the malaria parasites, our goal is to contribute to the development of effective vaccines that can reduce the global burden of malaria, particularly in regions most affected by this disease.
Our comprehensive approach includes advanced immunological studies, innovative vaccine design, and collaboration with international research partners to ensure the broad applicability and impact of our findings.
Image showing the intra-erythrocytic stages of malaria parasite:
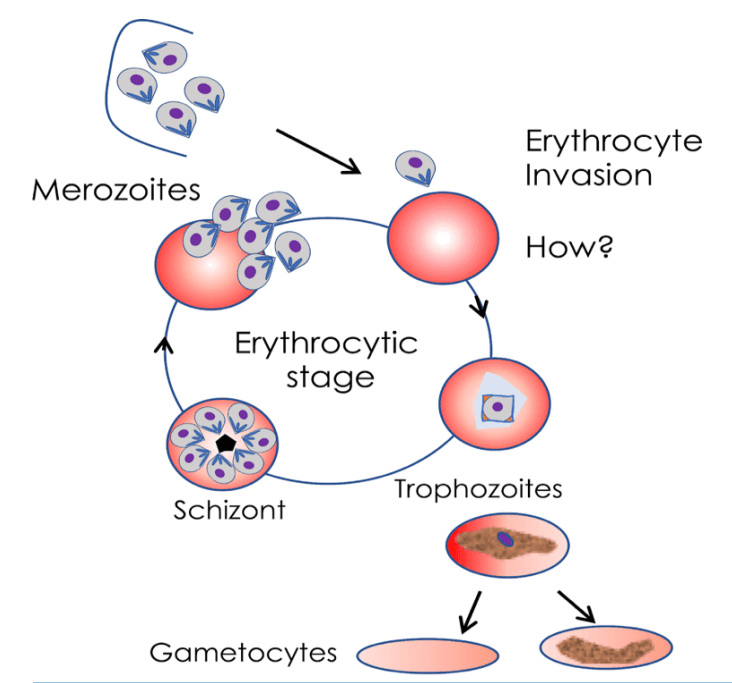
Key Achievements:
- Genome-Wide Identification: Conducted a comprehensive genome-wide identification of several novel blood-stage malaria vaccine candidates of P. falciparum using a functional approach based on malaria genome information.
- Detailed Characterization: Performed detailed characterization of these novel blood-stage vaccine candidates, enhancing our understanding of their potential as effective vaccines.
- Identification of Proteins/Epitopes: Successfully identified proteins and epitopes for further downstream characterization as vaccine molecules.
Our work significantly contributes to the development of new malaria vaccines, offering promising candidates for expedited testing and potential deployment in malaria-endemic regions.
2. Improving Maternal and Neonatal Health
a. Understanding Infections in Pregnancy to Enhance Health Outcomes
Our research examines how malaria during pregnancy impacts maternal immunity, placental function, and newborn health. By combining proteomics, biomarker discovery, and clinical studies, we are uncovering pathways that link infection to poor outcomes such as low birth weight and preterm delivery. We are committed to translating these insights into maternal vaccines, better diagnostics, and health system strategies to protect mothers and their babies.
b. Vaccine Antigens Against Pregnancy-Associated Malaria
One of our key projects, “Systematic prioritization and initial characterization of Plasmodium falciparumvaccine antigens against pregnancy associated malaria”, is part of the EDCTP2 programme supported by the European Union.
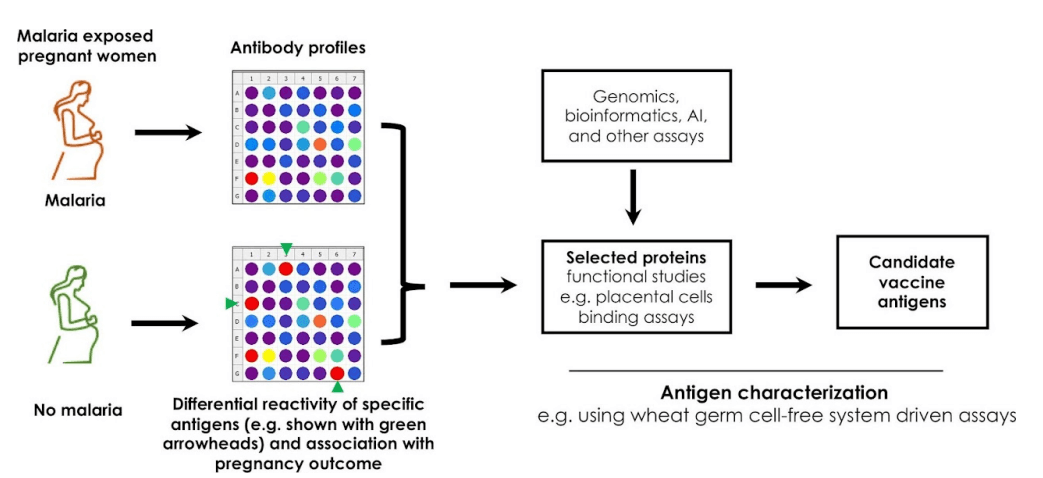
Malaria in pregnancy contributes to over 10,000 maternal and 200,000 infant deaths per year worldwide, with over 25 million pregnancies at risk of infection in sub-Saharan Africa alone. Many of these deaths are due to severe anaemia in pregnant women and low birth weight. Malaria infection is highest between 13 and 18 weeks of pregnancy, when infected red blood cells begin to accumulate in the placenta causing placental malaria infection. However, there is strong scientific evidence that women in their second and subsequent pregnancies have a lower risk of placental malaria infection and severe malarial disease partly due to antibodies they acquire against Plasmodium falciparum (P. falciparum) parasite proteins. By identifying these protein targets, we can develop vaccines that can protect women against malaria at this critical stage of raising a family.
The objective of this study therefore, is to identify proteins against which pregnant women raise antibodies that protect them against placental malarial infection in pregnancy-associated malaria. In previous malaria vaccine studies, the focus has been on a limited number of protein targets lacking the full depth of available genomic sequence information. Here, a eukaryotic protein expression system will be used to produce a large library of P. falciparum proteins selected based on gene expression, biophysical characteristics such as size, 3D structure among others and the genomic diversity criteria that includes different protein families. We are investigating the ability of the proteins selected to cause antibody production that blocks infected red blood cells from binding to the placenta. The aim is to understand the mechanism of antibody inhibiting the parasite while introducing protein diversity at the earliest stage of target identification; a key aspect of vaccine studies.
Antibodies with the highest functional ability to block iRBCs will be tested in combination for future development, and further validation of vaccine targets. This approach will radically change the scale and pace of pregnancy-associated vaccine development, and will produce resources of broad utility to the malaria research community and strengthen research in Kenya and Africa.
We recently summarized our thoughts in this review.
3. Emerging Infectious Diseases, Antimicrobial Resistance, and Diagnostics Innovation
We are at the forefront of developing rapid, sensitive molecular assays and genomic surveillance platforms to address antimicrobial resistance, STIs, and emerging pathogens such as SARS-CoV-2. Our studies range from hospital wastewater monitoring of AMR genes to advancing CRISPR- and nanopore-based diagnostic technologies. With a strong focus on pandemic preparedness, we are committed to building locally relevant, globally impactful solutions for future health threats.
4. Collaborative Approach to Science
We believe groundbreaking science is a team effort. Our scientific platforms and shared resources are designed to foster innovation and accelerate discovery with our local and global network of partners.
Immunological Platform
Our ELISA and Luminex facilities provide robust, high-throughput serological analysis to dissect immune responses and evaluate vaccine efficacy in collaborative studies. We also produce recombinant proteins and serological tools for immunological research.

Cell Culture Platform
We conduct and support genetic studies and functional analysis through our dedicated facilities for gene cloning and malaria parasite transfection, enabling key mechanistic investigations.


Sequencing Platform
Harnessing the power of Illumina's NextSeq 1000 and Oxford Nanopore technology, we offer comprehensive genomic solutions for pathogen surveillance and evolution studies.


Biobanking Facilities
Our biobank is a vital repository, securely storing diverse clinical and field samples—from human cells and placental tissue to parasite isolates—to fuel collaborative research projects.


Translating Science Into Impact
We advance discovery and ensure our research directly informs malaria control, maternal health, and infectious disease preparedness globally.
Connect with Us